Demineralisers
Ion-exchanging demineralisers
Exploit the resins' ability to selectively react with anions and cations. The resins withhold positive and negative ions from the water, binding with them and in exchange releasing a radical.
Ion-exchanging demineralisers can be se- parated into:
· twin column separate bed demineralisers with or without degassing tower
· single column mixed bed demineralisers.
How they work
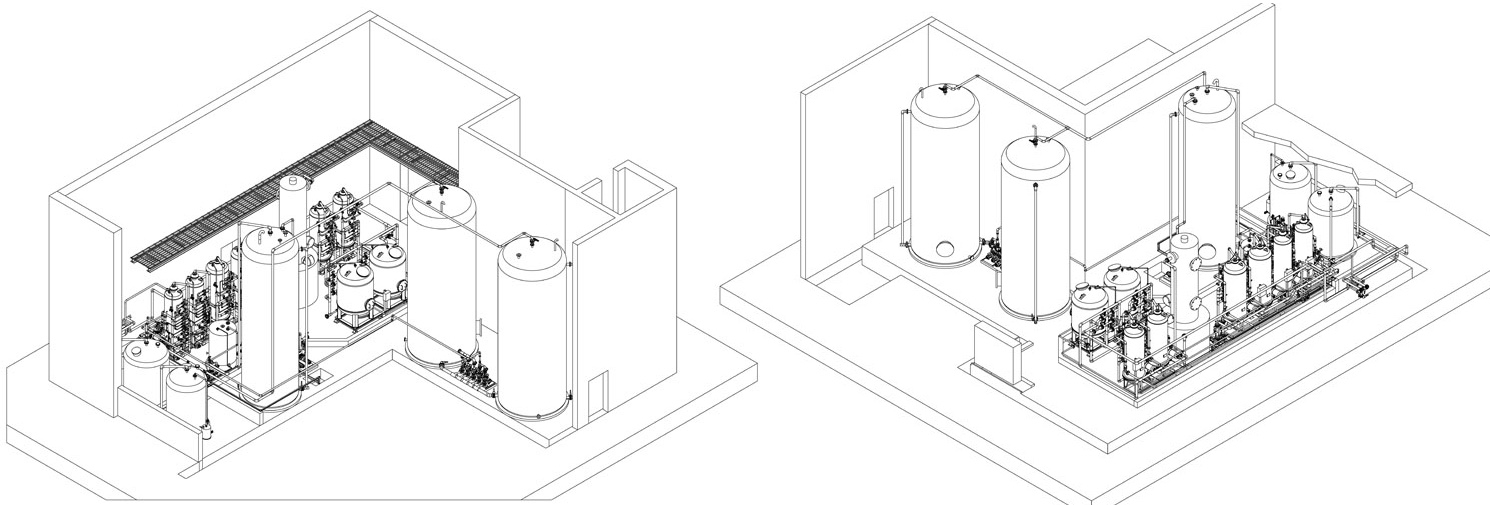
The demineralisers are made up of a primary chamber, the so-called cationic exchanger, in which the metallic ions react with the resins, binding with them and being substituted by hydrogen atoms, as in the example:
CaSO4 + 2 H+ ------> H2SO4 + Ca++
Thus, highly acidic water is obtained, which is basically free of metallic ions. It must be noted that the exchanger is la- belled cationic as the cations remain in the water;
If many bicarbonates are present in the feed water, the following reaction takes place:
NaHCO3 + H+ ------> Na+ + CO2 + H2O
As the carbon dioxide can be separated by physical means, when possible the wa- ter coming from the cationic exchanger is sent to a separation tower, in which the CO2 is separated in a counter-flow air current.
The water coming from the cationic exchanger, if necessary degassed, then passes into the anionic exchanger, where the acid radicals are exchanged with hydroxyls -OH, and the reaction is the following:
H2SO4 + 2 OH- ------> 2 H2O + SO4--
Thus the acid radicals are removed, resulting in pure water.
In some cases, the cation and anion resins are mixed and the treatment takes place in a single chamber.
This type of apparatus is called "mixed bed" and is less efficient in terms of mass exchange, but far superior in terms of final purity; in plants above a certain dimension, a mixed bed is added after the classic demineraliser.
Regeneration
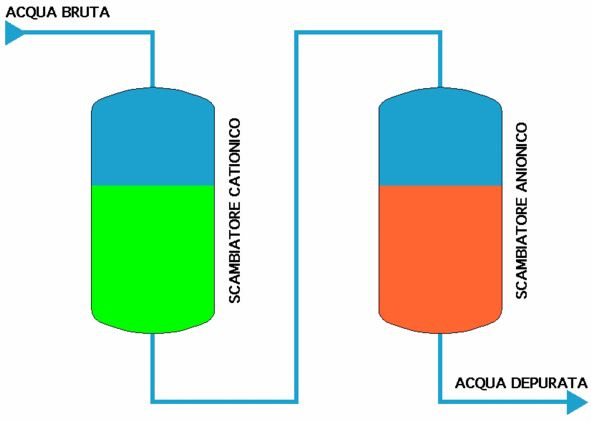
The resins, cationic and ionic, have a certain number of radicals available to exchange; when theseare used up, the exchange ceases. However, the radicals can be reconstituted via a chemical regeneration process.
After being rinsed in a counter-current to remove any solids suspended or resin powders ground in the work phase, the resins are put in contact with an active solution:
for the cationic resin, a strong acid solution (usually hydrochloric acid, sometimes sulphuric acid or rarely another kind) and the following reaction takes place, presuming that calcium has been removed:
++2HCl------>CaCl2+2H+
while for the anionic resin, a strong base (usually caustic soda, sometimes ammonia) gives the following reaction, in the same hypothesis:
SO4-- + 2 NaOH ------> Na2SO4 + 2 OH-
Thus where hydrochloric acid HCl and caustic soda NaOH are used, an eluate com- posed of various metal chlorides and various sodium salts is obtained.
Areas of application
Demineralising plants can be used wherever water of a high degree of purity is required:
· Galvanizing processes
· Circuit cleaning for electronics
· The food industry
· Hospitals, laboratory analysis, the pharmaceutical industry
· Cogeneration turbines




